





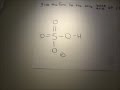










3) Identify the acid, base, conjugate acid, and conjugate base of HSO4 + NH3 -----> SO4 + NH4 Acid: HSO4 Base: NH3 Conjugate acid: NH4 Conjugate base: SO4 Got the wrong answer? Click Here to review Click Here to go back to the QUIZ!! 4) Identify the acid, base, conjugate acid, and conjugate base of C2H3O2 + HCl -----> C2H4O2 + Cl Acid: HCL Base Answer to Identify the conjugate base for each acid. conjugate base of H2SO, conjugate base of HSO4: conjugate base of NH : Identi... Conjugate Base of H 2. . S O4. . H 2. . S O4. . −H + → H S O4−. H2SO4 is the chemical name for sulfuric acid, and the conjugate base is hydrogen sulfate. Even though HSO4 is also an acid, it can either be a conjugate acid or a conjugate base depending on context. A conjugate base is not necessarily a basic molecule. Mar 2, 2014. H 2SO4 sulfuric acid will donate an H + in solution to form H 3O+ hydronium. The remaining H SO− 4 would be the conjugate base of this dissociation. I hope this was helpful. SMARTERTEACHER. H2SO4 is an acid so it donates a proton and after donating a proton it will be HSO4- H2SO4 = HSO4- + H+ HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton. The conjugate base of HSO4- is SO4 (2-), if that’s the question that you are asking. Bronsted acid Conjugate base. HF F- H 2 SO4 Related questions 0 votes. 1 answer. What will the conjugate bases for the following Bronsted acids: HF , H2SO4 HCO3 - and NH4 + asked Jun 2, 2018 in Chemistry by Golu (106k points) equilibrium; class-11 +1 vote. 1 answer. The species: H2O, HCO3- , HSO4-, and NH3 can act both as Brönsted acids and bases. asked Oct 7, 2017 in Chemistry by jisu Question: Give The Formula Of The Conjugate Base Of HSO4- . Give The Formula For The Conjugate Acid Of HSO4-. This problem has been solved! See the answer. Show transcribed image text. Videos. Step-by-step answer 04:16 0 0. Expert Answer 100% (54 ratings) Previous question Next question A conjugate base is what is left when an acid loses a proton (H+), for example the conjugate base of sulfuric acid (H2SO4) is the bisulfate ion (HSO4-). A conjugate acid is the product of a base The given species H2SO4 is an acid so it is suppose to donate a proton and after donating a proton it will be HSO4 -. H2SO4 <---------> HSO4 - + H +. Now, HSO4 - is a base since it has the ability to accept a proton but it is a conjugate base to H 2 SO 4 since it is formed by the H2SO4 after donating a proton.
[index] [3437] [1758] [6938] [1927] [1899] [4879] [2721] [7] [2179] [1142]
Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... How does Fluoroantimonic Acid react to an iPhone 7? I decided to find the answer! FACEBOOK:https://www.facebook.com/techraxTWITTER:https://twitter.com/techra... This is Part-III of Acids & Bases in which following points are explained in an interesting way: Sulphuric acid (H2SO4) Physical & chemical properties of H2SO4, Uses of H2SO4 Hydrochloric acid ... 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... Determine acid/base ratio of a buffer - Duration: 12:43. Peter Klappa 38,107 views. 12:43. Using the HH equation to calculate partial charges on amino acids - Duration: 5:26. In this video we will look at the equation for H2SO4 + H2O and write the products. There are two reactions we need to consider since both of the hydrogens...
Copyright © 2024 best.tabking.online